- About the Department
- Faculty Profile
- Courses Offered
- PO and CO
- Certificate / Add-on courses
- Teaching-learning
- Result and Student Progression
- Departmental Activities and Achievements
- Collaborative activities under MoU
- Alumni
- Photo Gallery
The Chemistry Department is one of the oldest departments of Sammilani Mahavidyalaya. The Department was founded on the year of 1998, at the very beginning of the College. Then Chemistry was taught as an elective general subject and the Honours course has been introduced at 2010 under the affiliation to University of Calcutta.
The Department has three well equipped laboratories to pursuing practical offered in CBCS (Choice Based Credit System) under University of Calcutta. Inorganic Chemistry laboratory and Organic Chemistry laboratory are well maintained and organized with all the chemicals, glassware, Melting point and Boiling point apparatus, Vacuum pump, Water bath etc. needed for pursuing entire practical mentioned in the syllabus of CBCS (Choice Based Credit System) under University of Calcutta. Physical Chemistry laboratory is equipped with all the modern instruments (UV-Vis Spectrophotometer, Digital Polarimeter, Digital Potentiometer, Digital pH meter, Conductivity meter etc.) required for pursuing the entire practical offered under CBCS (Choice Based Credit System). The Department has several Computers with net connection to perform all the basic computer programming languages like FORTRAN and MS-Excel. Printers are also connected with the computers to take the print out of the experimental results.

Prof. Chandan Kumar Jana
Designation: Principal
Email: principal.sammilani@gmail.com
Phone Number: 8100598811Get Detail »

Dr. Shefali Pal (Laha)
Designation: Faculty
Email: shefalipal62@gmail.com
Phone Number: 9433679003Get Detail »
Dr. Chittaranjan Chanda (Honorary)

Sri. Sumit Pal
Designation: SACT
Email: sumitpal2005@gmail.com
Phone Number: 9836057211Get Detail »

Dr. Senjuti Banik
Designation: SACT
Email: senjuti.banik@gmail.com
Phone Number: 8910314125
Get Detail »

Dr. Durba Ganguly
Designation: SACT
Email: durbaganguly.ju@gmail.com
Phone Number: 8240486586Get Detail »

Dr. Krishnendu Aich
Designation: SACT
Email: krishnendu.aich8@gmail.com
Phone Number: 8240936101Get Detail »

Sri Sandipan Mallik
Designation: SACT
Email: sandipanmallick3@gmail.com
Phone Number: 7003753296Get Detail »
PROGRAMME AND COURSE OUTCOME OF CHEMISTRY HONOURS
(UNDER CBCS)
Course Outcomes
| Semester | Course Code | Course Outcomes |
|
SEM – 1 |
CC-1 (INORGANIC & ORGANIC CHEMISTRY) |
CO – 1 · To know extra nuclear structure of atom · To understand acid base reactions in Inorganic Chemistry · To know the basic concepts of redox reactions · To understand the basic concepts of organic chemistry based on chemical bonding and physical properties · To learn the basic reaction mechanism of Organic Chemistry · To study the estimation of ions or salts by acid-base titration method and oxidation-reduction titration method by hand-on practical · To learn experimentally about the separation of compounds from a solid binary mixture by using common laboratory reagents |
|
CC-2 (PHYSICAL & ORGANIC CHEMISTRY) |
CO – 2 · To understand the basic concept of kinetic theory of gases and know how to solve numerical problems related to that topic. · To learn the transport processes of liquids and gases. · To understand the kinetics of the reactions · To learn the basic concepts of Stereochemistry of Organic molecules · To study about the formation and stability of reaction intermediates · To study the kinetics of decomposition of H2O2, acid-catalyzed hydrolysis of methyl acetate, viscosity measurement of unknown liquids, measurement of solubility of sparingly soluble salts. · To know experimentally how to determine the boiling points of organic liquid compounds. |
|
|
SEM – 2 |
CC-3 (ORGANIC CHEMISTRY) |
CO – 3 · To learn stereochemistry of chiral compounds arises due to presence of chiral-axis; concept of prostereoisomerism and study of conformational analysis of some acyclic organic molecules. · To understand reaction kinetics, reaction thermodynamics and tautomerism of organic compounds. · To know reaction mechanisms of nucleohilic substitution reactions and elimination reactions. · To learn experimentally how to synthesize, calculate the yield percentage and determine melting point of pure organic compounds in the laboratory. |
|
CC-4 (INORGANIC CHEMISTRY) |
CO – 4 · To learn about the basic concepts and types of chemical bonding, laws, rules and equations for formation of chemical bonds, solubility, hybridization and dipole moment of molecules. · To study the modern approaches of chemical bonding (Molecular Orbital Theory, Metallic Bonding concept, Role of weak intermolecular forces). · To understand about the concept of radioactivity and radioactive compounds, nuclear reactions, artificial radioactivity, radio carbon dating, hazards of radiation and safety measures. · To know experimentally how to estimate the percentage of chlorine in bleaching powder; vitamin C; arsenic and antimony in a sample by iodimetric titration method. Students can also learn how to estimate Cu in brass, Cr and Mn in steel and Fe in cement. |
|
|
SEM – 3 |
CC-5 (PHYSICAL CHEMISTRY) |
CO – 5
· To learn in detail about the first and second laws of Chemical Thermodynamics and the related terms; to get idea about thermo-chemistry and thermodynamic relationships and system of variable compositions. · To gain vast knowledge on chemical equilibrium and electrochemistry. · To learn experimentally how to do the potentiometric and conductometric titrations of different compositions, determine the Ka of weak acid and heat of neutralization of a strong acid by a strong base. |
|
CC-6 (INORGANIC CHEMISTRY) |
CO – 6
· To study in detail about modern periodic table, physical and chemical properties of the elements along a group or period, factors influences those properties, relativistic effects and inert pair effect. · To study the chemistry of s and p block elements including noble gases and their compounds in detail. · To learn about inorganic polymers in detail. · To know the meaning of various terms involved in co-ordination chemistry, Werner’s theory for complex formation, structural and stereoisomerism of co- ordination complexes. · To learn the complexometric and gravimetric estimation of different ions, chromatographic separation of (i) Ni(II) and Cu(II) ions, (ii) Fe(III) and Al(III) ions. |
|
|
CC-7 (ORGANIC CHEMISTRY) |
CO – 7
· To learn in detail about the synthesis, properties, and reaction mechanisms of alkenes and alkynes · To understand about different types of electrophilic and nucleophilic aromatic substitution reactions, reaction intermediates and their mechanisms. · To study the properties and reactions of carbonyl compounds and corresponding reaction mechanisms. · To learn preparations, reactions and corresponding reaction mechanisms of organometallic compounds. · To study experimentally the qualitative detection solid and liquid organic compounds. · To learn experimentally the quantitative estimation of some organic compounds by titration method. |
|
|
SEC-A |
CSECA – 2
SEC-A-2. ANALYTICAL CLINICAL BIOCHEMISTRY
· Helps to understand about the preparation, structures, reactions and biological importance of carbohydrates, proteins, enzymes, lipids and lipoproteins. · To know the biochemistry of different diseases through a diagnostic approach by blood and urine analysis. |
|
|
SEM – 4 |
CC-8 (ORGANIC CHEMISTRY) |
CO – 8
· To understand in detail about the synthesis, separation, properties, identification, chemical reactions and their corresponding mechanism of nitrogen containing compounds. · Discussion about different kinds of rearrangement reactions. · Helps to know the logic of organic synthesis · To study UV-Visible, IR and 1H NMR spectroscopy in detail. · Helps to know experimentally the qualitative analysis of single solid organic compounds and know to prepare derivatives of functional groups. |
|
CC-9 (PHYSICAL CHEMISTRY) |
CO – 9
· Helps to understand about the applications of Thermodynamics in Colligative Properties and Phase Equilibrium · To study the fundamentals of Quantum Mechanics · Helps to know the Bravais Lattice and Laws of Crystallography, Crystal Planes and Specific Heat of Solid · To know experimentally how to study phase diagram of a Phenol-Water system, kinetic study of inversion of cane sugar, determination of partition co-efficient value, pH of an unknown solution and pH metric titration of an acid against strong base. |
|
|
CC-10 (INORGANIC CHEMISTRY) |
CO – 10
· Helps to understand about the structures, stability, colour, magnetism and Orgel diagram of the co-ordination compounds on the basis of modern concepts of chemical bonding. · To study the chemical and physical properties of d and f Block elements and their compounds. · To learn the reaction kinetics and mechanisms of inorganic reactions. · To study experimentally how to synthesize inorganic complexes and determine the λmax values of inorganic complexes. · To calculate the 10Dq value by spectrophotometric method. |
|
|
SEC – B |
CSECB – 3
SEC-B-3. PHARMACEUTICALS CHEMISTRY
· Helps to understand about the drug discovery, design and development of representative drugs of the following classes: Antipyretic, Analgesics, Anti- inflammatory, Anti-bacterial, Antifungal, Antiviral, Antibiotics, Anti-laprosy, Central Nervous System agents, HIV-AIDS related drugs · To know about aerobic and anaerobic fermentation, importance of Vitamins and Amino acids, synthesis of Penicillin, Cephalosporin, Chloromycetin, Streptomycin and their role as an antibiotic.
CSECB – 4
SEC-B-4. PESTICIDE CHEMISTRY
· Helps to understand about the preparation, structures, properties, reactions, benefits and adverse effects of pesticide compounds
|
|
|
SEM-5 |
CC-11 (PHYSICAL CHEMISTRY) |
CO – 11
· Helps to understand the fundamental concept, basic terms, derivation and application of Quantum Mechanics · To know about the necessary laws, rules, terms, expressions and derivations statistical thermodynamics · To learn laws, rules and equations for numerical analysis of Roots of Equation and Least-Squares Fitting. · To study about the Computer Programming on Roots of equation, Numerical differentiation and Numerical integration. |
|
CC-12 (ORGANIC CHEMISTRY) |
CO – 12
· To learn in detail about the synthesis, properties, chemical reactions and reaction mechanisms of polynuclear hydrocarbons and their derivatives. · To study the chemical reactions, properties and synthesis of heterocyclic compounds. · To know in detail about the stereochemistry, properties and chemical reactions of alicyclic compounds. · To learn the mechanism, stereochemistry and regioselectivity of pericyclic reactions. · Helps to understand about the classification, structure, properties, reactions and use of carbohydrate molecules. · Deals with the synthesis, structure, properties, chemical and biological reactions of amino acids, peptides and nucleic acids. · To learn experimentally how to separate molecules by chromatographic methods · To study how to analyze the Organic compounds by spectroscopic techniques. |
|
|
DSE |
CDSEA – 2
A-2. APPLICATIONS OF COMPUTERS IN CHEMISTRY
· Helps to understand about the basics of computer programming (FORTRAN), creating and application of spreadsheet software (MS Excel) · Helps to know about statistical data analysis. · To learn how to prepare graphs by using spreadsheet, help to determine vapour pressure, rate constant, equilibrium constant, molar extinction coefficient value, concentration of ions at equilibrium and molar enthalpy of vapourisation. · To study about the Acid-Base Titration Curve, Plotting of First and Second derivative Curve for pH metric and Potentiometric titrations, Calculation and Plotting of a Precipitation Titration Curve with MS Excel, Michaelis-Menten Kinetics for Enzyme Catalysis using Linear and Non – Linear Regression.
CDSEB – 1
B-1. INORGANIC MATERIALS OF INDUSTRIAL IMPORTANCE
· Helps to understand about the manufacture, properties, compositions, classes and applications of industrially important materials such as ceramics, glasses, cements, fertilizers, surface coating materials and batteries. · To know about alloys, manufacture of steel, composition and properties of different types of steels. · To learn about the general principles, properties, classification, industrial use, deactivation and regeneration of catalysis. · Helps to understand about the preparation and explosive properties of organic and inorganic explosives and the basic idea of rocket propellant. · To learn how to analyze the composition of cement, composition of percentage of metals in alloy, electroless metallic coatings on ceramic and plastic. · To know how to determine free acidity in ammonium sulphate fertilizer, estimation of Calcium in Calcium ammonium nitrate fertilizer and phosphoric acid in superphosphate fertilizer. |
|
|
SEM-6 |
CC-13 (PHYSICAL CHEMISTRY) |
CO – 13
· To study the Theoretical Principles in Qualitative Analysis · To learn about Bioinorganic Chemistry and Organometallic Chemistry · To know about the catalytic role of organometallic compounds in different types of industrial processes. · To study experimentally the qualitative detection of known and unknown radicals and insoluble materials in a mixture. |
|
CC-14 (INORGANIC CHEMISTRY) |
CO – 14
· To learn in detail about molecular spectroscopy. · To understand about the basic principles and laws of Photochemistry and also get idea about the theory of reaction rate. · To know details about surface energy and surface tension; Classification, Adsorption Isotherms and applications of Adsorption; Classification, rules and properties of Colloids. · To learn about the fundamental concepts, important equations, properties and applications of polarizability and dipole moment. · To know how to determine surface tension of a liquid; Indicator constant of an acid base indicator; pH of an unknown buffer solution and CMC of a micelle experimentally. · To study the kinetics of K2S2O8 + KI reaction and Verification of Beer and Lambert’s Law for KMnO4 and K2Cr2O7 solution experimentally |
|
|
DSE |
CDSEA – 3
A-3. GREEN CHEMISTRY AND CHEMISTRY OF NATURAL PRODUCTS
· To learn about green chemistry and its necessity. · To study about the principles of green chemistry and designing the green synthetic routes. · To know about the examples of green reactions and future trends in green reaction. · To learn the synthesis, psychological properties, isolation medicinal importance and other synthetic use of terpenes and alkaloids · To learn how to perform green synthesis of a number of organic compounds in the laboratory.
CDSEB – 3
B-3. POLYMER CHEMISTRY
· To learn about the history, classification and functionality of polymeric materials. · To know about the kinetics of polymerization, details on crystallization and morphology of crystalline polymers, determination of crystalline melting point of a crystalline material and the factors effecting crystalline melting point. · To understand the nature and structure of polymers, determination of molecular weight of polymers and thermodynamics of polymer solution. · To study the preparation, structure, properties and application of different types of addition and condensation polymers. · To know how to prepare polymers by using free radical polymerization, redox polymerization, interfacial polymerization, precipitation polymerization, addition polymerization and condensation polymerization process. · To learn experimentally how to characterize and analyze a polymeric compound or material.
CDSEB – 4
B-4. DISSERTATION
· To know how to do research work and write a review article on a particular field/topic as assigned by the supervisor/guide · To know how to present the research works or the review article. |
PROGRAM OUTCOMES (UNDER CBCS)
- PO1: Ready the graduate students for their career in government job, in industry and to motivate them for higher education and to take up research as a career.
- PO2: The student will be capable of applying modern technologies, handling advanced instruments and Chemistry related soft-wares to identify formulate and analyze complex scientific problems for chemical analysis and characterization of materials.
- PO3: The various branches of Chemistry can be helpful to graduate students to develop critical thinking and to design, carry out, record and analyze the results of chemical reactions also expose the diversified aspects of chemistry where the students experience a broader outlook of the subject that.
- PO4: The course curriculum has been designed to provide opportunity to act as team player by contributing in laboratory, field based situation, industry and also think methodically, independently and draw a logical conclusion.
- PO5: Employ critical thinking and the scientific knowledge to design, carry out, record and analyze the results of chemical reactions.
- PO6: Create an awareness of the impact of chemistry on the environment, society, and development outside the scientific community.
- PO7: A Chemistry graduate student learn how to follow the green routes for the synthesis of chemical compounds and also find out new greener routes for sustainable development. The course also helps them to understand the causes of environmental pollution and thereby applying environmental friendly policies instead of environmentally hazard ones in every aspect.
- PO8: Chemistry graduate students can handle many decent instruments and advanced technologies to synthesize, characterize and analyze the chemical compounds very skillfully. Such a wonderful practice in the graduate level will bring a good opportunity to the students for getting job in industries besides academic and administrative works
PROGRAMME SPECIFIC OUTCOME (PSO):
PSO-1: The students pursuing graduation in chemistry are expected to gain knowledge of the fundamental concepts of chemistry and applied chemistry through theory and practical. The students would have to develop in depth understanding of various aspects chemistry.
PSO-2: Graduates in Chemistry are expected to demonstrate, understanding and achieve critical thinking ability of the fundamental principles, including scientific reasoning to solve problems, of organic chemistry, inorganic chemistry, analytical chemistry and physical chemistry.
PSO-3: Students are expected to possess sufficient knowledge how to synthesize a chemical compound and perform necessary characterization and develop analytical abilities for independent thinking.
PSO-4: Chemistry students are expected to be well trained which will help student’s to build-up a progressive and successful career in Chemistry.
PSO-5: Graduates are expected to be team players, with productive co-operations involving members from diverse socio-cultural backgrounds.
PSO-6: Students will demonstrate research skills in chemistry, including proper laboratory notebook and record keeping skills, recognizing hazards, minimizing risks, and safe laboratory practices.
MAPPING OF CO, PO, PSO
|
PO1
|
PO2 | PO3 | PO4 | PO5 | PO6 | PO7 | PO8 | PSO-1 | PSO-2 | PSO-3 | PSO-4 | PSO-5 | PSO-6 | |
| CO1 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||||
| CO2 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||||
| CO3 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||||||
| CO4 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||||||
| CO5 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||||||||
| CO6 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||||||||
| CO7 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||||||
| CO8 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||||||||
| CO9 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||||||
| CO10 | ✔ | ✔ | ✔ | ✔ | ✔ | |||||||||
| CO11 | ✔ | ✔ | ✔ | ✔ | ||||||||||
| CO12 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||||||
| CO13 | ✔ | ✔ | ✔ | ✔ | ✔ | |||||||||
| CO14 | ✔ | ✔ | ||||||||||||
| CSECA – 2 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||||||
| CSECB – 3 | ✔ | ✔ | ✔ | |||||||||||
| CSECB – 4 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||||||
| CDSEA – 2 | ✔ | ✔ | ✔ | ✔ | ✔ | |||||||||
| CDSEB – 1 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||||||
| CDSEA – 3 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||||
| CDSEB – 3 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||||||||
| CDSEB – 4 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
ATTAINMENT TROUGH DIRECT METHODS
|
Sl. No. |
Direct Assessment Method
|
Assessment frequency |
Description |
|
1 |
Internal Assessment Test |
Once in a semester |
Internal Assessment is held for 20% of the total marks of each paper/subject/module i.e. 20 marks for a paper of full marks 100. 50% of the total marks i.e. 10 marks is assigned to Internal Assessment is assessed on the basis of Internal Examination and remaining 50% i.e. 10 marks is assessed based on the class attendance
|
|
2 |
Class Test
|
As per requirement
|
It is a method used to continuously assess the student’s understanding capabilities |
|
3 |
End Semester Examination
|
Once in a semester
|
End Semester examination (theory or practical) are centrally held by the University to assess the learning ability and theoretical knowledge of the student. End Semester Examinations are held ordinarily at the end of the concerned Semester, i.e., Semester-I, Semester-III, Semester-V in December-January and Semester-II, Semester-IV, Semester-VI in June-July.
|
|
4 |
Practical Semester Examination
|
Once in a semester
|
End Semester practical examinations assess whether all the students have acquired the practical applications of the particular subject by hands on tests. Examinations are held along with the theoretical schedule.
|
|
5 |
Presentations
|
As per requirement
|
Presentations are conducted to assess student’s communication and presentation skills along with depth of the subject knowledge in the form of seminars, debates or group discussions.
|
|
6 |
Attendance
|
As per CU rules
|
Total 10 Marks is allotted for all CBCS courses: The marks obtained in every course from Class Attendance by the students have the following manner. 6 marks for attending 60% or above but less than 75% of the number of lectures delivered, 8 marks for attending 75% or above but less than 90% of the number of lectures delivered, 10 marks for attending 90% or above of lectures delivered, and such attendance are calculated from the date of commencement of classes or date of admission whichever is later) |
Department of Chemistry has been organized a 30 hours add-on course on “ Basic safety Practices in Chemistry Laboratory ” from 4th November 2022 to 12th April 2023. All the classes were taken by our departmental teachers Dr. Shefali Pal, Prof. Sumit Pal, Dr. Durba Ganguly, Dr. Krishnendu Aich, Dr. Senjuti Banik, Prof. Sandipan Mallik. Fourteen students from 3rd semester chemistry advanced and pure science –Bio science general actively participated in this curriculum. This course was inaugurated by our respected Teacher-in -Charge, Dr Sharmila Chakraborty. She has delivered her motivational speech about the importance and relevance of this course. Regular laboratory safety training course covered the fundamentals of laboratory safety rules. Students gathered knowledge about common toxic chemicals and safety measures during handling them. We have also conducted some practical classes in order to give them proper practical knowledge about various functions of laboratory equipments. The students showed their attentions and curiosity during the time of theoretical and practical classes. Their eagerness and enthusiasm made us satisfied and the desired outcome of the curriculum was successful.
- Mentor- mentee system
As per Semester strength, students are distributed as mentees, who are supposed to share and interact with their respective mentor. In most cases, mentees face problem in understanding various topics. In those situations, mentors take extra classes, advise the mentees to follow good reference books (from departmental and central library), encourage in group discussions. Each mentee is also encouraged to attend regular classes, do practical classes regularly and address the issue within the class itself. Besides, if mentee reports of any personal/ family issues that are hampering their academics, mentors try to solve those issues providing rational and mental support. Regular parent teacher meetings and constant mentoring of the students are regularly done to understand their shortcomings and provide solutions to the same.
- Remedial classes
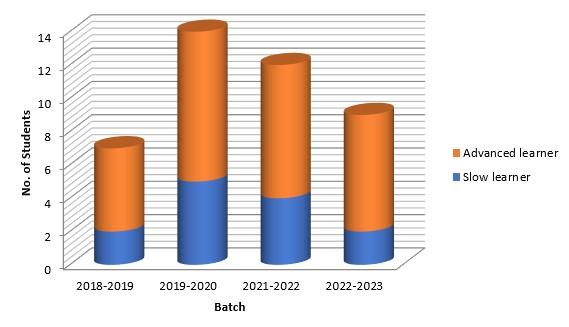
Extra classes (apart from regular classes) are offered when necessary. Additional materials are provided for better learning and understanding. Students are identified as advanced and slow learners through continuous internal evaluation, and remedial or extra classes are provides as and when required.
- Participative and experiential learning (seminars, workshops, wall magazines, poster presentation, educational tour etc.) :
- Seminars
1)
Title of the seminar : International Year of Periodic Table of Chemical Elements.
Date : 27th March , 2019
Name of the speaker/s :
- J. P. Naskar
Dept. of Chemistry , Jadavpur University
Topic : International Year of Periodic Table of Chemical Elements.
Poster Presentation by Chemistry Hons. Students
- Aniruddha Das
Topic : History of the Periodic Table
- Chandrani Pramanik
Topic : Introduction of Modern Periodic Table
- Anamika Das
Topic : Unusual facts about some elements in Periodic Table
- Ayan Nath
Topic : 5 Interesting molecules of Periodic Table
2)
Title of the seminar : “Synthetic polymers : A Boon or a Bane”
Date : 23rd March, 2022 Wednesday;
Name of the speaker/s :
Prof. Dipankar Chattopadhyay,
Dept. of Polymer science and technology,
University of Calcutta
Poster Presentation by Chemistry Hons. Students
Seminar report :
The department of Chemistry of Sammilani Mahavidyalaya organized a one day college level seminar entitled “Synthetic polymers: A Boon or a Bane” on 23rd March, 2022 Wednesday from 12 noon to 3 pm. One eminent speaker from Dept. of Polymer science and technology, University of Calcutta named, Prof. Dipankar Chattopadhyay delivered a valuable popular lecture to the present audience.
3)
Title of the seminar : “Dissertation Project Presentation”
Date : 14th June, 2023 Wednesday
Seminar report :
The department of Chemistry of Sammilani Mahavidyalaya organized a departmental student seminar on 14th June, 2023 Wednesday from 12 noon to 3 pm. All the students of 6th Semester Chemistry Honours students presented their Dissertation Projects works ( which were review works of different topics of research papers ) in front of the faculty members of various departments of our college. The students have represented their project work through power point presentation. The seminar was an interactive session through which the students have faced many questionnaires’ and by answering these questions they have share their thoughts and knowledge with the experts.
The main purpose of this seminar was to enable students to improve their knowledge and understanding of the topics. Far from the textbook and academic syllabus , the students have learnt more new topics that would boost their confidence and productivity in research work.
- Wall Magazines
Students of all the semesters prepare wall magazines with different relevant topics related to their subjects. These wall magazines are displayed in our notice boards.
- NMR Visit to Jadavpur University
The Department organizes Laboratory Visit to Department of Chemistry, Calcutta University or Jadavpur University, Kolkata every year. The primary aim of the event is to imbibe the undergraduate students with the impetus for future research, thereby encouraging them in the process. Moreover, NMR practical is included in the CBCS Syllabus of Semester IV [ CC-4-8 (Th.) ] & Semester V [ CC-5-12 (Pr.) ] , which is beyond our facility in the college. So the students can achieve practical exposure of the instruments which is helpful for their understanding of theory and practical both.
Evaluation systems:
The internal assessment of CU examinations for each semester and class tests are part of our regular evaluation system. In 1st semester there is a system to take an Entry level assessment test to evaluate their basic knowledge in Chemistry and to distinguish between advanced and slow learners.
Internal assessment dates and record of marks:
Academic session – 2022-2023
| Date of internal evaluation | Semester | Course | Topic | Full marks | Taken by |
Pattern of question (online/offline) |
| 23/06/2023 | VI | CC-13 | Bio-Inorganic Chemistry | 10 | Dr. Shefali Pal | M.C.Q. , Offline |
| 23/06/2023 | VI | CC-14 | Molecular Spectroscopy | 10 | Dr. Durba Ganguly | M.C.Q. , Offline |
| 23/06/2023 | VI | DSE-A3 | Green Chemistry | 10 | Dr. Krishnendu Aich | M.C.Q. , Offline |
| 28/07/2023 | IV | CC-8 | Organic Chemistry | 10 | Mr . Sandipan Mallik | M.C.Q. , Offline |
| 28/07/2023 | IV | CC-9 | Physical Chemistry | 10 | Dr. Durba Ganguly | M.C.Q. , Offline |
| 28/07/2023 | IV | CC-10 | Inorganic Chemistry | 10 | Dr. Shefali Pal | M.C.Q. , Offline |
| 28/07/2023 | IV | SEC-B2 | Pesticide Chemistry | 10 | Mr . Sandipan Mallik | M.C.Q. , Offline |
| 09/08/2023 | II | CC-4 | Inorganic Chemistry-II | 10 | Mr. Sumit Pal | M.C.Q. , Offline |
| 09/08/2023 | II | CC-3 | Organic Chemistry-II | 10 | Dr. Krishnendu Aich | M.C.Q. , Offline |
Academic session – 2021-2022
| Date of internal evaluation | Semester | Course | Topic | Full marks | Taken by |
Pattern of question (online/offline) |
| 29/06/2022 | VI | DSE-B3 | Polymer Chemistry | 10 | Mr . Sandipan Mallik | M.C.Q. , Offline |
| 29/06/2022 | VI | DSE-A3 | Green Chemistry | 10 | Dr. Krishnendu Aich | M.C.Q. , Offline |
| 29/06/2022 | VI | CC-14 | Molecular Spectroscopy | 10 | Dr. Durba Ganguly | M.C.Q. , Offline |
| 29/06/2022 | VI | CC-13 | Bio-Inorganic Chemistry | 10 | Dr. Shefali Pal | M.C.Q. , Offline |
| 13/01/2023 | V | CC-11 | Physical Chemistry | 10 | Dr. Senjuti Banik | M.C.Q. , Offline |
| 13/01/2023 | V | DSE-B1 | Industrial Chemistry | 10 | Mr. Sumit Pal | M.C.Q. , Offline |
| 13/01/2023 | V | DSE-A2 | Computer Application | 10 | Dr. Durba Ganguly | M.C.Q. , Offline |
| 27/07/2022 | IV | CC-8 | Organic Chemistry | 10 | Mr . Sandipan Mallik | M.C.Q. , Offline |
| 27/07/2022 | IV | CC-9 | Physical Chemistry | 10 | Dr. Durba Ganguly | M.C.Q. , Offline |
| 27/07/2022 | IV | CC-10 | Inorganic Chemistry | 10 | Dr. Shefali Pal | M.C.Q. , Offline |
| 27/07/2022 | IV | SEC-B2 | Pesticide Chemistry | 10 | Mr . Sandipan Mallik | M.C.Q. , Offline |
| 17/01/2023 | III | CC-5 | Physical Chemistry | 10 | Dr. Senjuti Banik | M.C.Q. , Offline |
| 17/01/2023 | III | CC-6 | Inorganic Chemistry | 10 | Mr. Sumit Pal | M.C.Q. , Offline |
| 17/01/2023 | III | CC-7 | Organic Chemistry | 10 | Dr. Krishnendu Aich | M.C.Q. , Offline |
| 17/01/2023 | III | SEC-A2 | Analytical Biochemistry | 10 | Mr . Sandipan Mallik | M.C.Q. , Offline |
| 27/07/2022 | II | CC-3 | Organic Chemistry-II | 10 | Dr. Krishnendu Aich | M.C.Q. , Offline |
| 27/07/2022 | II | CC-4 | Inorganic Chemistry-II | 10 | Mr. Sumit Pal | M.C.Q. , Offline |
| 21/06/2022 | I | CC-1 | Inorganic + Organic | 10 | Dr. Shefali Pal + Dr. Krishnendu Aich | M.C.Q. , Offline |
| 21/06/2022 | I | CC-2 | Physical + Organic | 10 | Mr . Sandipan Mallik + Dr. Senjuti Banik | M.C.Q. , Offline |
Academic session – 2020-2021
| Date of internal evaluation | Semester | Course | Topic | Full marks | Taken by |
Pattern of question (online/offline) |
| 02/08/2021 | VI | DSE-B3 | Polymer Chemistry | 10 | Mr . Sandipan Mallik | M.C.Q. , Online |
| 04/08/2021 | VI | CC-13 | Bio-Inorganic Chemistry | 10 | Dr. Shefali Pal | M.C.Q, Online |
| 05/08/2021 | IV | CC-8 | Organic Chemistry | 10 | Mr . Sandipan Mallik | M.C.Q. , Online |
| 11/08/2021 | IV | CC-10 | Inorganic Chemistry | 10 | Dr. Shefali Pal | M.C.Q. , Online |
| 13/08/2021 | IV | SEC-B1 | Pharmaceutical Chemistry | 10 | Mr. Sumit Pal | M.C.Q. , Online |
| 14/08/2021 | II | CC-4 | Inorganic Chemistry-II | 10 | Mr. Sumit Pal | M.C.Q. , Online |
| 31/08/2021 | II | CC-3 | Organic Chemistry-II | 10 | Dr. Krishnendu Aich | M.C.Q. , Online |
| 11/03/2022 | I | CC-2 | Physical + Organic | 10 | Mr . Sandipan Mallik + Dr. Senjuti Banik | M.C.Q. , Offline |
Academic session – 2019-2020
| Date of internal evaluation | Semester | Course | Topic | Full marks | Taken by |
Pattern of question (online/offline) |
| 19/03/2021 | V | DSE-A2 | Computer Application | 10 | Dr. Durba Ganguly | M.C.Q. , Online |
| 22/03/2021 | V | DSE-B1 | Industrial Chemistry | 10 | Mr. Sumit Pal | M.C.Q. , Online |
| 24/03/2021 | V | CC-12 | Organic Chemistry | 10 | Dr. Krishnendu Aich | M.C.Q. , Online |
| 24/03/2021 | V | CC-11 | Physical Chemistry | 10 | Dr. Senjuti Banik | M.C.Q. , Online |
| 24/03/2021 | III | SEC-A1 | Mathematics And Statistics | 10 | Mr. Sumit Pal | M.C.Q. , Online |
| 09/03/2021 | III | CC-7 | Organic Chemistry | 10 | Dr. Krishnendu Aich | M.C.Q. , Online |
| 09/03/2021 | III | CC-5 | Physical Chemistry | 10 | Dr. Senjuti Banik | M.C.Q. , Online |
| 22/03/2021 | III | CC-6 | Inorganic Chemistry | 10 | Dr. Shefali Pal | M.C.Q. , Online |
| 24/03/2021 | I | CC-2 | Physical + Organic | 10 | Mr . Sandipan Mallik | M.C.Q. , Online |
| 24/03/2021 | I | CC-1 | Inorganic + Organic | 10 | Dr. Shefali Pal | M.C.Q. , Online |
| 22/12/2020 | IV | SEC-B1 | Pharmacetical chemistry | 10 | Mr. Sumit Pal | M.C.Q. , Online |
Academic session – 2018-2019
| Date of internal evaluation | Semester | Course | Topic | Full marks | Taken by |
Pattern of question (online/offline) |
| 29/11/2019 | III | SEC-A2 | Analytical Biochemistry | 10 | Mr . Sandipan Mallik | M.C.Q. , Offline |
| 29/11/2019 | III | CC-7 | Organic Chemistry | 10 | Dr. Krishnendu Aich | M.C.Q. , Offline |
| 29/11/2019 | III | CC-6 | Inorganic Chemistry | 10 | Dr. Shefali Pal | M.C.Q. , Offline |
| 29/11/2019 | III | CC-5 | Physical Chemistry | 10 | Dr. Durba Ganguly | M.C.Q. , Offline |
| 03/12/2019 | I | CC-1 | Inorganic + Organic | 10 | Dr. Shefali Pal | M.C.Q. , Offline |
| 03/12/2019 | I | CC-2 | Physical + Organic | 10 | Mr . Sandipan Mallik | M.C.Q. , Offline |
| 06/06/2019 | II | CC-4 | Inorganic Chemistry-II | 10 | Mr. Sumit Pal | M.C.Q. , Offline |
| 06/06/2019 | II | CC-3 | Organic Chemistry-II | 10 | Dr. Krishnendu Aich | M.C.Q. , Offline |
- Final Results of Batches (Academic sessions: 18-19, 19-20, 20-21, 21-22, 22-23):
|
Roll-No |
Name |
Marks obtained |
CGPA |
Grade |
||||||
| Sem I | Sem II | Sem III | Sem IV | Sem V | Sem VI | |||||
| 203513-21-0006 | Dip Mukherjee | 348 | 353 | 470 | 345 | 238 | 308 | 7.77 | A | |
| 203513-21-0137 | Bhagaban Naskar | 354 | 360 | 470 | 378 | 288 | 316 | 8.27 | A+ | |
| 203513-21-0147 | Swapnil Ghosh | 346 | 355 | 461 | NA | NA | 274 | NA | NA | |
| 203513-21-0115 | Utsav Mondal | 359 | 356 | 454 | 330 | 236 | 295 | 7.67 | A | |
| 203513-21-0058 | Gopal Saha | 353 | 364 | 460 | 344 | 240 | 297 | 7.79 | A | |
| 203513-21-0151 | Rakesh Mondal | 307 | 324 | 445 | NA | NA | NA | NA | NA | |
| 203513-11-0072 | Dolan Mondal | 363 | 370 | 463 | 354 | 240 | 299 | 7.905 | A | |
| 183513-21-0011 | Danish Ameen | NA | NA | 445 | 347 | NA | 287 | NA | NA | |
| 203513-11-0069 | Shreya Jana | 362 | 371 | 462 | 322 | NA | NA | NA | NA | |
RESULTS OF BATCH 2022-23
RESULTS OF BATCH 2021-22
|
Roll-No |
Name |
Marks obtained |
CGPA |
GRADE |
||||||
| Sem I | Sem II | Sem III | Sem IV | Sem V | Sem VI | |||||
| 193513-11-0002 | Ankita Jana | 289 | 322 | 431 | 441 | 371 | 269 | 8.14 | A+ | |
| 193513-11-0003 | Anushree Chakraborty | 276 | 327 | 427 | 442 | 367 | 273 | 8.071 | A+ | |
| 193513-11-0005 | Jhuma Mazumdar | 281 | 337 | 432 | 443 | 377 | 266 | 8.16 | A+ | |
| 193513-11-0006 | Krishna Saw | 302 | 316 | 427 | 441 | 364 | 257 | 8.09 | A+ | |
| 193513-21-0001 | Soumyadip Dinda | 254 | 313 | 416 | 435 | 362 | 256 | 7.76 | A | |
| 193513-21-0002 | Ayan Das Ghosh | 297 | 311 | 410 | 435 | 357 | 251 | 7.848 | A | |
| 193513-21-0003 | Ritesh Khatua | 263 | 307 | 428 | 442 | 351 | NA | NA | NA | |
| 193513-21-0004 | Aritra Dey | 259 | 315 | 404 | 428 | 354 | 257 | 7.67 | A | |
| 193513-21-0005 | Sangeet Chakraborty | 299 | 312 | 414 | 432 | 357 | NA | NA | NA | |
| 193513-21-0006 | Sunny Bose | 279 | 291 | 379 | 395 | 335 | NA | NA | NA | |
| 193513-21-0007 | Diganta Halder | 296 | 307 | 414 | 434 | 360 | NA | NA | NA | |
| 193513-21-0117 | Amit Aich | 313 | 316 | 412 | 439 | 356 | 263 | 8.047 | A+ | |
RESULTS OF BATCH 2020-21
|
Roll-No |
Name |
Marks obtained |
CGPA |
GRADE |
||||||
| Sem I | Sem II | Sem III | Sem IV | Sem V | Sem VI | |||||
| 183513-11-0002 | Chandrani Pramanik | 262 | 250 | 332 | 397 | 351 | 372 | 7.60 | A | |
| 183513-11-0003 | Aditi Saxena | 264 | 246 | 307 | 370 | 353 | 365 | 7.35 | A | |
| 183513-21-0002 | Asish Kumar Khatua | 250 | 274 | 285 | 385 | 352 | 368 | 7.39 | A | |
| 183513-21-0005 | Abhash Kayal | 203 | 253 | 355 | 379 | 342 | 353 | 7.29 | A | |
| 183513-21-0006 | Argha Karmakar | 260 | 265 | 306 | 394 | 341 | 366 | 7.38 | A | |
| 183513-21-0007 | Aniruddha Das | 263 | 278 | 305 | 379 | 343 | 371 | 7.452 | A | |
| 183513-21-0008 | Pabitra Maity | 230 | 266 | 299 | 373 | 348 | 358 | 7.18 | A | |
| 183513-21-0009 | Ayan Nath | 243 | 254 | 309 | 397 | 340 | 351 | 7.27 | A | |
| 183513-21-0013 | Subha Ghorai | 227 | 269 | 263 | 385 | 338 | 364 | 7.069 | A | |
| 183513-21-0015 | Soumen Chhatui | 258 | 254 | 272 | 391 | 348 | 369 | 7.232 | A | |
| 183513-21-0016 | Debdutta Mondal | 258 | 266 | 285 | 362 | 346 | 361 | 7.1 | A | |
| 183513-21-0171 | Atish Modak | 268 | 273 | 323 | 405 | 349 | 373 | 7.569 | A | |
| 183115-21-0272 | Kingshuk Patra | 247 | 253 | 360 | 372 | 347 | 353 | 7.319 | A | |
- Progression to higher education
STUDENT PROGRESSION
Batch of 2023 (Year of Passing)
| Name | Progression after B. Sc. | |
| 1 | Gopal Saha | M.Sc. |
| 2 | Bhagaban Naskar | M.Sc. |
| 3 | Dip Mukherjee | M.Sc. |
| 4 | Dolan Mandal | M.Sc. |
Batch of 2022 (Year of Passing)
| Name | Progression after B. Sc. | |
| 1 | Ankita Jana | M.Sc. |
| 2 | Krishna Saw | M.Sc. |
| 3 | Anushree Chakraborty | M.Sc. |
| 4 | Jhuma Mazumder | M.Sc. |
Student Employment 2021-22 (Batch)
| Name | Batch | Organization, Position, Salary | |
| 1 | Anushree Chakraborty | 2022 | Indian Post |
Batch of 2021 (Year of passing)
| Name | Progression after B. Sc. | ||
| 1 | Atish Modak | M.Sc in IIT | |
| 2 | Soumen Chatui | M.Sc in IIT | |
| 3 | Asish Khatua | M.Sc. | |
| 4 | Aniruddha Das | M.Sc | |
| 5 | Kingshuk Patra | B.Ed. Tet qualified | |
| 6 | Chandrani Pramanik | M.Sc | |
| 7 | Debdutta Mondal | B.Ed. | |
| 8 | Pabitra Maity | B.Ed. Tet qualified | |
| 9 | Meghna Bhattachryya | M.Sc | |
Student Employment 2020-2021 (Batch)
| Name | Batch | Organization, Position, Salary | |
| 1 | Aditi Saxena | 2021 | Associate: Wipro |
| 2 | Argha Karmakar | 2021 | Analyst – Chemical Process : Elogix Software Pvt. Ltd. |
Batch of 2020 (Year of passing)
| Name | Progression after B. Sc. | |
| 1 | Utpalendu Nasker | B. Ed. |
| 2 | Amlan Halder | B. Ed. |
| 3 | Barun Dey | B. Ed. |
| 4 | Balaram Halder | B. Ed. |
| 5 | Subhadeep Barik | B. Ed. , CTET |
| 6 | Piyali Giri | B. Ed. , M.Sc. |
| 7 | Kartick Barik | B. Ed. , M.Sc. |
| 8 | Anamika Das | B.Ed. , Primary TET , CTET |
| 9 | Ankita Mondal | M.Sc |
| 10 | Sweety Shaw | M.Sc. |
Student Employment 2019-2020 (Batch)
| Name | Batch | Organization, Position, Salary | |
| 1 | Mahadev Mondal | 2020 | Chemist of Gold Testing Laboratory. P. C. Chandra Group |
| 2 | Balaram Halder | 2020 | Part Time job in a school |
| 3 | Kartick Barik | 2020 | Himadri Speciality Chemical Ltd. |
| 4 | Sweety Shaw | 2020 | Assistant Lecturer, Aakash Educational Services Ltd. |
- Collaborative initiatives under MOU:
|
Programme Name |
Date |
Resource Person / Conducting Institution |
No. of participants / Students |
Synopsis of the Programme ( provide link / photos )
|
| 2021-2022 | ||||
|
Celebration of World Environmental Day
|
06/06/2022 |
1) Dr. Chaitali Bhattacharjee Associate Professor , Dept. of Chemistry , Netajinagar College for Women
2) Charu Chand Hansda , Assistant Professor , Dept. of Chemistry , Netajinagar |
30 ( including students of all the semesters and staff of both the colleges ) |
Two faculty members of Netajinagar College for Women delivered lectures on the importance of World Environmental Day.
|
| 2022- 2023 | ||||
|
Faculty Exchange
|
23/09/2022 12/11/2022 25/11/2022 02/12/2022 |
Dr. Gitashri Naiya , Professor , Department of Chemistry Netajinagar College for Women |
09 Students of Semester 5 of Chemistry Hons. of Sammilani Mahavidyalaya |
Paper : CC5-11-P & DSE-A2-Th ( FORTRAN )
|
| Serial No | Session | Date | Alumni activities | Number of Participants | Brief Reports | Photos |
| 1 |
2020-21
|
1st May – 10th May, 2021 | Community help during COVID-19 period |
06 (6th Semester CEMA students of Sammilani Mahavidyalaya, Pass out: 2021) |
Distribution of foods, sanitizers, masks etc. essential items to poor and needy peoples. Dry ration, vegetables and kitchen being run in many places near Sundarban and Mousuni island. | Annexure – 1 |
| 2 |
2021-22
|
5th September, 2021 | Teachers’ day celebration | 25 (Including faculty members) | Online teachers’ day celebration through Google Meet during COVID period. | Annexure – 2 |
| 3 |
2022-23
|
25th November, 2022 |
‘Parampara’ – the Alumni Study Circle
|
05 students | Topic: Basic principle of co-ordination chemistry and how to solve university question papers. | Annexure – 3 |
| 4 |
2022-23
|
14th December, 2022 |
‘Parampara’ – the Alumni Study Circle
|
05 students | Topic: Chemical kinetics. He taught the basic concepts of chemical kinetics and how to solve the question papers for university examination and for JAM | Annexure – 3 |
| 5 |
2022-23
|
17th March, 2023 |
‘Parampara’ – the Alumni Study Circle
|
09 students | Interacted with our present students by providing necessary guidance for their carrier planning. The topic of the lecture is: How to crack competitive examination. | Annexure – 3 |
Serial 1: Our alumni students of department of Chemistry distributed foods, sanitizers, masks etc. essential items to poor and needy peoples during COVID period. Dry ration, vegetables and kitchen being run in many places near Sundarban and Mousuni island on 1st May to 10th May 2021.